Welcome to iGrow News, Your Source for the World of Indoor Vertical Farming
Marijuana Study Finds CBD Can Cause Liver Damage
Researchers at the University of Arkansas for Medical Science recently rolled up their sleeves to investigate CBD hepatotoxicity in mice.
June 18, 2019
Mike Adams Contributor
Vices I cover various facets of the cannabis culture.
Image Credit | Urine Drug Test
There is no denying that cannabidiol, more commonly referred to as CBD, is rapidly becoming more popular in the United States than sliced bread. It is a hot trend that got started several years ago after Dr. Sanja Gupta showed the nation in his documentary 'Weed 2' just how this non-intoxicating component of the cannabis plant was preventing epileptic children from having seizures.
Since then, CBD, a substance often touted as being safer than popping pills, has become highly revered as an alternative treatment for a variety of common ailments from anxiety to chronic pain. But a new study suggests that CBD may spawn its fair share of health issues. Specifically, scientists have learned that this substance could be damaging our livers in the same way as alcohol and other drugs.
Researchers at the University of Arkansas for Medical Science recently rolled up their sleeves to investigate CBD hepatotoxicity in mice. What they found was while this cannabis derivative is gaining significant recognition as of late in the world of wellness, people that use CBD are at an elevated risk for liver toxicity.
The findings, which were published earlier this year in the journal Molecules, suggest that while people may be using CBD as a safer alternative to conventional pain relievers, like acetaminophen, the compound may actually be just as harmful to their livers.
It is the methods used in this study that makes it most interesting.
First, researchers utilized all of the dosage and safety recommendations from a CBD-based drug known as Epidiolex. If this name sounds familiar, it should. Last year, the U.S. Food and Drug Administration approved it as a treatment for certain kinds of childhood epilepsy. It was a development that marked the first time in history that a cannabis-based medicine was approved for nationwide distribution in the United States.
Lead photo: Hemp oil, Hand holding bottle of Cannabis oil against Marijuana plant, CBD oil pipette. alternative remedy or medication,medicine concept | GETTY
Cannabidiol Is A Powerful New Antibiotic
New research has found that Cannnabidiol is active against Gram-positive bacteria, including those responsible for many serious infections (such as Staphyloccocus aureus and Streptococcus pneumoniae), with a potency similar to that of established antibiotics such as vancomycin or daptomycin
June 28, 2019
Summary: Cannabidiol (CBD), a non-psychoactive compound extract of marijuana, has the potential to be used as an antibiotic. Researchers found CBD was remarkably effective at killing a wide range of Gram-positive bacteria, including bacteria that are resistant to common antibiotics. Additionally, CBD does not lose its effectiveness after extended treatment.
Source: ASM
New research has found that Cannnabidiol is active against Gram-positive bacteria, including those responsible for many serious infections (such as Staphyloccocus aureus and Streptococcus pneumoniae), with a potency similar to that of established antibiotics such as vancomycin or daptomycin. The research is presented at ASM Microbe, the annual meeting of the American Society for Microbiology.
Cannabidiol, the main non-psychoactive chemical compound extracted from cannabis and hemp plants, has been approved by FDA for the treatment of a form of epilepsy and is being investigated for a number of other medical conditions, including, anxiety, pain and inflammation. While there is limited data to suggest Cannabidiol can kill bacteria, the drug has not been thoroughly investigated for its potential as an antibiotic.
Work led by Dr Mark Blaskovich at The University of Queensland’s Institute for Molecular Bioscience’s Centre for Superbug Solutions, in collaboration with Botanix Pharmaceuticals Ltd, an early stage drug discovery company investigating topical uses of synthetic cannabidiol for a range of skin conditions, found that Cannabidiol was remarkably effective at killing a wide range of Gram-positive bacteria, including bacteria that have become resistant to other antibiotics, and did not lose effectiveness after extended treatment.
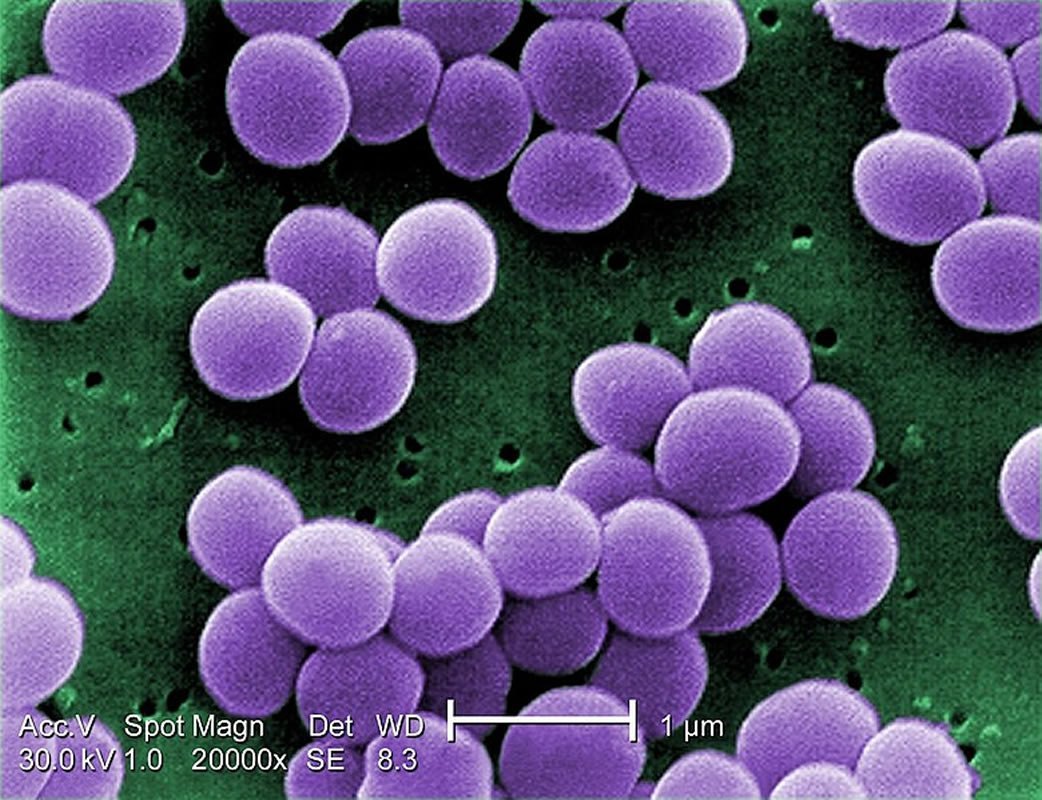
Importantly, the drug retained its activity against bacteria that have become highly resistant to other common antibiotics. Under a very high magnification of 20,000x, this scanning electron micrograph (SEM) shows a strain of Staphylococcus aureus bacteria taken from a vancomycin intermediate resistant culture (VISA). The image is credited to the CDC.
“Given cannabidiol’s documented anti-inflammatory effects, existing safety data in humans, and potential for varied delivery routes, it is a promising new antibiotic worth further investigation,” said Dr. Blaskovich.
“The combination of inherent antimicrobial activity and potential to reduce damage caused by the inflammatory response to infections is particularly attractive.”
Importantly, the drug retained its activity against bacteria that have become highly resistant to other common antibiotics. Under extended exposure conditions that lead to resistance against vancomycin or daptomycin, Cannabidiol did not lose effectiveness. Cannabidiol was also effective at disrupting biofilms, a physical form of bacteria growth that leads to difficult-to-treat infections.
The project was co-funded by Botanix and Innovation Connections, an Australian government grant scheme to commercialize new products, processes and services. The paper will be presented on Sunday June 23rd from 11am-1 pm at the annual conference of the American Society for Microbiology, ASM Microbe 2019, at the Moscone Convention Center in San Francisco.
ABOUT THIS NEUROSCIENCE RESEARCH ARTICLE
Source:
ASM
Media Contacts:
Aleea Khan – ASM
Image Source:
The image is credited to the CDC and is in the public domain.
Original Research: The findings were presented at ASM Microbe 2019 in San Fancisco, California.
Marijuana Study Finds CBD Can Cause Liver Damage
Researchers at the University of Arkansas for Medical Science recently rolled up their sleeves to investigate CBD hepatotoxicity in mice.
Hemp oil, Hand holding bottle of Cannabis oil against Marijuana plant, CBD oil pipette. alternative remedy or medication,medicine concept | GETTY
June 18, 2019
Mike Adams Contributor
Vices I cover various facets of the cannabis culture.
Image Credit | Urine Drug Test
There is no denying that cannabidiol, more commonly referred to as CBD, is rapidly becoming more popular in the United States than sliced bread. It is a hot trend that got started several years ago after Dr. Sanja Gupta showed the nation in his documentary 'Weed 2' just how this non-intoxicating component of the cannabis plant was preventing epileptic children from having seizures.
Since then, CBD, a substance often touted as being safer than popping pills, has become highly revered as an alternative treatment for a variety of common ailments from anxiety to chronic pain. But a new study suggests that CBD may spawn its fair share of health issues. Specifically, scientists have learned that this substance could be damaging our livers in the same way as alcohol and other drugs.
Researchers at the University of Arkansas for Medical Science recently rolled up their sleeves to investigate CBD hepatotoxicity in mice. What they found was while this cannabis derivative is gaining significant recognition as of late in the world of wellness, people that use CBD are at an elevated risk for liver toxicity.
The findings, which were published earlier this year in the journal Molecules, suggest that while people may be using CBD as a safer alternative to conventional pain relievers, like acetaminophen, the compound may actually be just as harmful to their livers.
It is the methods used in this study that makes it most interesting.
First, researchers utilized all of the dosage and safety recommendations from a CBD-based drug known as Epidiolex. If this name sounds familiar, it should. Last year, the U.S. Food and Drug Administration approved it as a treatment for certain kinds of childhood epilepsy. It was a development that marked the first time in history that a cannabis-based medicine was approved for nationwide distribution in the United States.
Many Unanswered Questions, Concerns About CBD Products, Says FDA Acting Chief At First Public Hearing
The acting head of the Food and Drug Administration launched the agency’s first hearing on CBD products Friday with a laundry of list of questions about cannabidiol, better known as CBD, which is already being sold in pills, tinctures, skin lotions, sodas and dog food
You can buy CBD in oils, supplements, soda, even dog food. But most of them violates federal food and drug regulations, prompting concerns over safety and deceptive marketing.
A jar of CBD gummy candies at The Cannabis World Congress & Business Exposition trade show in New York City. REUTERS/Mike Segar (Mike Segar/Reuters)
By William Wan
May 31, 2019
The acting head of the Food and Drug Administration launched the agency’s first hearing on CBD products Friday with a laundry of list of questions about cannabidiol, better known as CBD, which is already being sold in pills, tinctures, skin lotions, sodas and dog food.
How much of the cannabis extract is safe to consume daily, he asked at the jam-packed, all-day hearing at FDA headquarters in Silver Spring. How will it interact with other drugs? What if a consumer is pregnant? What is CBD’s effect on children? What happens if someone takes it over the course of years?
Acting Commissioner Norman “Ned” Sharpless said answers to most of those questions are still unknown despite the popularity of many CBD products.
“There are important reasons to generally prohibit putting drugs in the food supply,” Sharpless said. And cannabis extracts like CBD “are no exception.”
During the 10 hours of testimony that followed, hemp growers, start-up businesses, academic researchers and consumer advocates argued about how FDA should regulate the already booming CBD industry. Some demanded strict oversight. Others — especially companies with millions at stake — lobbied for looser regulation.
But the common theme among them all: FDA needs to figure out its rules sooner, rather than later.
Even though FDA’s regulations make adding CBD to food and supplements illegal, the CBD industry has exploded in recent years with thousands of unproven products flooding the market. Companies have trumpeted the compound’s alleged health benefits — claiming it can reduce anxiety, pain and insomnia and treat conditions from Parkinson’s disease to cancer. But almost all such claims lack rigorous scientific proof, prompting concern among health officials and scientists about safety and deceptive marketing.
Without clearly defined regulations, no one knows for sure how much CBD is in products available on the market, or how safely the chemical compound is being manufactured and incorporated into them.
“It’s a wild West kind of environment right now,” said Yasmin Hurd, a psychiatry professor at the Icahn School of Medicine at Mount Sinai in New York City, who has researched CBD for almost 10 years. “I’m inundated every day with patients wanting to know how much CBD they should take, which ones to buy. But we don’t know what’s in the stuff now being sold. . . . We’ve had this explosion without guidance to the public or regulation.”
How the FDA will choose to regulate the industry and how long it will need to figure that out remain unclear. Those prospective regulations have become a fierce battleground: More than 400 people and groups applied for a chance to speak at Friday’s hearing, with roughly 120 speaking slots parsed out.
CBD can be derived from the marijuana plant or hemp. Congress in December legalized hemp as part of the Farm Bill, clearing the way for industrial production of the nonintoxicating compound from that plant. But the FDA quickly made it clear to companies that while hemp was legal, CBD extract remained under government regulation. In recent months, the agency has sent warning letters to some companies that it said were “illegally selling CBD products that claimed to prevent, diagnose, treat, or cure serious diseases, such as cancer.”
Billions are at stake in how the FDA decides to regulate the compound, with business analysts projecting the industry could grow to be worth as much as $22 billion in the next five years.
Market research firm New Frontier Data estimated that sales of CBD products in the United States more than tripled between 2014 and 2017, to $367 million.
Retailers like CVS and Walgreens have announced plans to sell CBD lotions and creams. Food and beverage companies have eagerly jumped in, too, with burger chain Carl’s Jr. selling CBD-infused burgers.
At Friday’s hearing, hemp growers argued that the CBD market represented thousands of jobs and a booming source of growth for the economy. CBD retailers relayed anecdotal evidence of patients who found relief to longstanding medical problems in their products.
Health advocates spoke equally passionately about the dangers posed by an unfettered CBD market.
So far, the agency has approved only one CBD-based drug, Epidiolex, which treats severe forms of childhood epilepsy.
In a phone interview, Orrin Devinsky, a New York University researcher who helped develop the drug, said the government’s haphazard approach has been frustrating to scientists trying to help suffering patients.
“You have researchers having to struggle through enormous expense and obstacles to study CBD,” he said. “At the same time, you can walk down to your neighborhood bodega and buy a CBD soda off the shelf. The nation and marketplace are in a horribly confused state.”
So far, treating epilepsy is the only application for CBD supported by rigorous scientific data, Devinsky said. Though less rigorous, some promising findings have emerged for CBD’s possible effect on anxiety and inflammation.
“The thing we worry about is someone with cancer forgoing real treatment like chemo and taking CBD instead,” said Devinsky, a neurology professor at NYU.
At Friday’s hearing, scientists and consumer advocates also warned that for the thousands of CBD products being sold, there is little data to guide dosage levels, expiration dates, and manufacturing protocols to make sure they don’t also contain other elements like tetrahydrocannabinol, or THC, the main psychoactive component in marijuana, which has been found in some CBD products.
Whatever the FDA decides, many in the industry are pressing for it to move quickly.
“I’ve talked to beverage companies and they want to get into this space, but they are not interested in just throwing themselves headlong into an area without science and engagement with regulators,” said Coleen Klasmeier, a former FDA staff lawyer and now partner at law firm Sidley Austin.
“For years now, the agency’s position has been just to throw up their hands and say it’s a confusing issue,” said Daniel Fabricant, a former FDA official overseeing dietary supplements, who is now chief executive of the Natural Products Association representing the supplement industry.
In addition to Friday’s hearing, the FDA has set a deadline of July 2 for written comments on the issue. It also convened a working group led by Deputy Commissioner Amy Abernethy to explore ways CBD products might be sold legally, the impact of such products on public health, and whether new FDA rules or congressional legislation may be needed.
In a string of tweets on Friday, Abernerthy summed up main points she and FDA officials were hearing over and over at the meeting: “Key questions about product safety need to be addressed. Data are needed to determine safety thresholds for CBD...There are both positive supporters of cannabis-cannabis derived products including CBD and also concerned citizens worried that widely available products can be harmful.”
Now That Hemp And CBD Are Legal, What Comes Next For Food And Beverage?
…they still face regulatory obstacles from the Food and Drug Administration,…
AUTHOR Cathy Siegner
Dec. 20, 2018
Dive Brief:
Now that the 2018 Farm Bill is signed into law, hemp and its derivatives are no longer classified as controlled substances by the Food and Drug Agency, and can legally be regulated by state and tribal governments and commercialized in foods and dietary supplements, New Hope Network reported.
U.S. hemp and CBD companies are expected to pursue listings on Nasdaq and other exchanges soon, according to Bloomberg. However, they still face regulatory obstacles from the Food and Drug Administration, the New Hope Network noted, because the agency's position has been that CBD can't be legally sold in conventional foods or dietary supplements.
While hemp and marijuana are both members of the cannabis family, hemp extracts contain CBD, a non-psychoactive compound that doesn't produce marijuana's trademark high from THC. Hemp does contain very low levels of THC, but hemp products legally sold in the U.S. must have no more than 0.3% of the chemical.
Dive Insight:
Besides removing hemp from the list of controlled substances, the new Farm Bill also expands research into commercial uses for the plant. Currently, hemp ingredients such as CBD oil, powders and seeds are being used to infuse beverages such as iced tea and are being added to a wide variety of other foods, including ice cream, salads, milk and even children's cereal.
The market for CBD and hemp products is already significant and likely to become more so. According to the Capital Press, a New Frontier Data report found that U.S. CBD sales jumped almost 40% in 2017, hitting $367 million. And, according to the Agricultural Marketing Resource Center, the total retail value of all U.S. hemp products last year was estimated at $820 million.
Major food and beverage makers are keeping an eye on the trend and considering how they might adapt forms of THC and CBD to their brands. Bloomberg recently reported that Coca-Cola has been talking with a Canadian cannabis company about marijuana-infused beverages following that country's recent nationwide legalization. A Coca-Cola spokesman told Bloomberg News the company has made no decision yet but is closely watching the growth of CBD as an ingredient in global functional wellness beverages.
THC, CBD and non-psychoactive terpenes from cannabis have appearing in beverages in states where such products are legal. Last year, Heineken-owned Lagunitas Brewing debuted a non-psychoactive, cannabis-flavored IPA brewed with terpenes — organic compounds that give plants their flavors — and the company introduced a THC-based sparkling water this year.
It's not a big stretch to combine cannabis and hops in beer brewing since they are genetically related. The key ingredient they share are terpenes. It's another question whether beer products containing both ingredients will spark a nationwide trend, although with hemp no longer a controlled substance, there could be many more drinks with some form of CBD or THC showing up on shelves and in coolers. Just this week, AB InBev and marijuana grower and distributor Tilray announced a joint $100 million investment to research cannabis-infused nonalcoholic beverages.
Other recent product introductions containing CBD are a nutrition bar from California-based SNAAK Bar that markets itself as optimizing sports performance — and is only available in California and online — and Spring's line of CBD-infused sodas sold in New York, Florida, Nevada, and Illinois.
One big unknown for this market is how the FDA will handle regulating hemp and CBD in products since the agency states that "it is a prohibited act to introduce or deliver for introduction into interstate commerce any food (including any animal food or feed) to which THC or CBD has been added." But because of the new Farm Bill's action regarding hemp, it's possible the agency will institute a rulemaking process to realign its regulation and enforcement of the crop and its products. Dave Donnan, a senior partner in A.T. Kearney's food and beverage practice, told Food Dive the regulatory machine will probably be figuring out how to make CBD a safe and legal ingredient.
“There's all these tactical things that 2019 will be the year to work through,” Donnan said.
Some concerns stem from the cannabis industry's poor food safety record and a tendency to market some edible and supplement products as miracle cures. The FDA took action last year against four firms selling supplements based on marijuana that were advertised as curing cancer. Such claims may not be as likely with CBD products, although the oil is said to help with pain, inflammation, anxiety, depression, insomnia and seizures, among other conditions. Critics say these benefits aren't based on science, but advocates counter there has been plenty of recent research to bolster health claims.
Whether hemp and CBD will be appearing across the board in foods and beverages is unclear at this stage since the ink is barely dry on the new Farm Bill. But as research grows into more commercial applications and companies innovate with new products testing public interest — and FDA's tolerance under its current policies — there could be a newly crowded segment attracting investors, retailers and consumers.
Despite Hemp Legalization, FDA Will Still Consider CBD Products Largely Illegal
FDA maintains hemp oil is a drug ingredient, and requires approval for products.
Published: Dec 23, 2018
Reuters
By ASSOCIATEDPRESS
SEATTLE — The hemp industry still has work ahead to win legal status for hemp-derived cannabidiol, or CBD oil, as an ingredient in food or dietary supplements despite the big farm bill President Donald Trump signed last week designating hemp as an agricultural crop.
CBD oils have become increasingly popular in lotions, tinctures and foods, but their legal status has been murky and the Food and Drug Administration has sent warning letters to some companies making health claims for CBD.
In a statement following Thursday’s bill signing in Washington, FDA Commissioner Scott Gottlieb restated his agency’s stance that CBD is a drug ingredient and therefore illegal to add to food or health products without approval from his agency.
“Selling unapproved products with unsubstantiated therapeutic claims is not only a violation of the law, but also can put patients at risk, as these products have not been proven to be safe or effective,” Gottlieb wrote.
CBD is a non-psychoactive compound found in hemp, a version of the cannabis plant that is low in THC, the part of cannabis that gives pot its high.
An FDA-approved drug for the treatment of seizures, Epidiolex, contains cannabis-derived CBD. GW Pharmaceuticals’ GWPH, +3.72% syrup became the first prescription drug derived from the cannabis plant in June.
The FDA statement also specified parts of hemp that are safe as food ingredients, but the CBD stance disappointed advocates. Courtney Moran, a lobbyist for Oregon hemp farmers, said she plans to work with U.S. Sen. Ron Wyden, an Oregon Democrat, to nudge the FDA toward greater acceptance of CBD.
“We do hope the FDA does clear a pathway for these products that have already hit store shelves and are out in the marketplace,” Moran said. She said it’s an “opportunity for industry to educate the FDA.”
The FDA statement said three ingredients derived from hemp — hulled hemp seeds, hemp seed protein and hemp seed oil — are safe as foods and won’t require additional approvals, as long as marketers do not make claims that they treat disease.
Hemp, like marijuana, already was legal in some states before Trump signed the farm bill. But now hemp farmers will be able to buy crop insurance, apply for loans and grants, and write off their business expenses on their taxes like any other farmer.
Mums To Marijuana: Pequannock, New Jersey, Family Farm Applies For License To Grow Medical Pot
Jai Agnish, North Jersey Record
2018
(Photo: Jai Agnish/Northjersey.com)
Ken VandeVrede, of Gro-Rite Garden Center & Florist and CEO of Hillview Med in Pequannock discusses applying for license to farm medical marijuana. Jai Agnish, Staff Writer, @jaiagnish
Fifty years ago the VanderVrede family delivered tomatoes from the farm in Pequannock to customers in Paterson. If the state allows, the family may soon replace the tomatoes with medical marijuana.
Gro-Rite Garden Center & Florist has applied for one of six available state licenses to grow medical marijuana on its 150 acres of farmland in Pequannock and Belvidere under the name Hillview Med.
"It's very competitive," said Ken VandeVrede, the CEO of Hillview Med in Pequannock.
The farming family is among 147 statewide applicants, 49 in North Jersey, including Evergreen Cultivation in West Milford, who want to cultivate, process, and sell medical marijuana. Two licenses will be awarded in North Jersey, two in Central Jersey and two in South Jersey. There are currently six active state licences in circulation.
The family sees the connection to Paterson as a natural one, VanderVrede said, after all that is where his grandfather started off delivering his tomatoes in the early days of the business after immigrating from Holland.
The New Jersey Board of Health is expected to announce which applicants will receive a license on Nov. 1.
"It would be absolutely amazing," VanderVrede said of landing one of the coveted licenses. "For us to get in on the ground floor of the medical cannabis market in New Jersey would be amazing."
Why medical marijuana?
The Hillview Med proposal results from Gov. Phil Murphy’s early-summer move to double the state’s marijuana providers to meet growing demand. With more than 28,000 registered patients by early July, the state program is on track to double its numbers in 2018, state records show.
"We consider this as the next evolving space in the agriculture space," VandeVrede said.
Hillview Med is also trying to be ready if recreational marijuana is legalized in the state, VanderVrede said. A bill to create a legal marijuana marketplace in New Jersey is nearly complete.
Based on what's happened in other states, medical marijuana growers will likely be grandfathered in and have a foot in the door to grow legal recreational marijuana, VandeVrede said.
The distinction between medicinal versus recreational use marijuana is determined at the consumer end, VanderVrede explained. It's just taxed differently.
"We feel we have a very, very strong application," VandeVrede said.
The move from growing mums to marijuana could be made in seven months, he said.
Gro-Rite ships hundreds of thousands of plants and flowers on a weekly basis to supermarkets including ShopRite, Whole foods, and others in a number of states including Virginia and Pennsylvania.
Among the products are hydroponic basil, which is grown in state-of-the-art greenhouses. The company has 750,000 square feet of greenhouse space on the two farms and is permitted to build 2.2 million square feet more. That's the equivalent of 13 football fields with the potential for 38 more, he said.
"We can expand quickly," VandeVrede said.
The other plus, the CEO said, is the farm can get an affordable product to market.
Hillview Med can produce a pound of marijuana for under $400, VanderVrete said on Thursday. He said applicants who plan to open a warehouse and use electricity will find it difficult to get below $1,000 a pound.
"The lowest cost producers are the ones with high-tech greenhouses," he said.
These are computer- and sensor-based systems to control temperature, humidity, and water metrics. Also, the use of a sealed, closed-loop temperature system avoids having to vent out stinky marijuana odors during production.
"I don't have many neighbors but I wouldn't even want to go there," VandeVrede said.
In addition to sustainably cultivated agriculture products, Gro-Rite's food and herb lines are certified organic. This approach would carry over to marijuana even though VanderVrede said certified organic cannabis is not a thing yet.
"We know how to grow food at the highest level that there is, so for us to move into the cannabis space, this is automatic production that we do already," he said.
It's a vertical integration license meaning Hillview Med would grow the plant, extract it into oils, edibles, pills, vapes, and distribute products to dispensaries.
Ken VandeVrede, of Gro-Rite Garden Center & Florist, and CEO of Hillview Med in Pequannock. (Photo: Courtesy of Ken VandeVrede)
Why the Paterson market?
Hillview Med chose Paterson for several reasons, one being the family's history of delivering produce to the "Silk City" going back 50 years. More recently Gro-Rite has provided fresh produce to food banks there and has forged relationships with community groups. He said the company would hire Paterson residents to work in the dispensary.
"We've done a lot of exciting stuff in Paterson," he said.
Another reason is the diversity of the city's population. Having a marijuana dispensary in an urban city helps fulfill the licensing goal to promote inclusivity with minorities, VandeVrede said.
Hillview Med/Gro-Rite Garden Center & Florist in Pequannock applied for a license to grow and distribute medical marijuana. (Photo: Jai Agnish/Northjersey.com)
Gro-Rite's expansion into the cannabis market would be a remarkable change for the family, said VandeVrede during a recent tour of the farm. He reminisced about when he worked on the Pequannock farm as a child. His father was in charge then and he expanded to farm into Belvidere.
VandeVrede, a non-active private pilot, said it would be fun to one day fly a helicopter from nearby Lincoln Park Airport to the grass strip at the Belvidere farm. Such a costly flight may not be so out of reach if his company secures one of the lucrative medical marijuana farming licenses.
Staff writer David M. Zimmer contributed to this article.
Follow Jai Agnish on Twitter: @JaiAgnish. Email: agnish@northjersey.com.